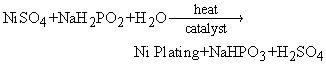Electrolessplating
Introduction
Autocatalytic (Electroless) plating, also known as electroless plating, is a plating process which involves deposition without any current applied. The process is a chemical reaction and is autocatalytic.
The deposition rate is normally 12.5 - 25 µm (.0005 - .001 in). Although, it has been done upto 650 µm (.026 in) in thickness, the coating is usually less than 50 µm (.002 in) in practice due to the slow deposition rate. The plating thickness tends to be uniform compared to electroplating due to the absence of electric fields and the associated problems in making them uniform.
Typically nickel and copper are used in electroless platings. In the case of nickel, the deposits are dense, relatively hard (43 - 55 HRC, increase to ~65 HRC after 2 hr. at 343ºC (650ºF) and brittle. Electroless Nickel is not as bright as electroplated, easy to solder and braze, but difficult to weld.
Autocatalytic platings are widely used for machine frames, base plates, fixtures, some machine parts where metal-to-metal wear applications are needed and the conventional oils and greases can not be used.
Theory of Autocatalytic Platings
In autocatalytic platings, the metal ion is reduced to a metal only on a specific surface, which must have a catalyst present before the reaction can begin.
The electroless plating involving a nickel sulfate bath has the reaction of