Electroplating
Introduction
Electroplating is an electrochemical process by which metal is deposited on a substrate by passing a current through the bath.
Usually there is an anode (positively charged electrode), which is the source of the material to be deposited; the electrochemistry which is the medium through which metal ions are exchanged and transferred to the substrate to be coated; and a cathode which is the substrate (the negatively charged electrode) to be coated.
Plating is done in a plating bath which is usually a non-metallic tank (usually plastic). The tank is filled with electrolyte which has the metal, to be plated, in ionic form.
The anode is connected to the positive terminal of the power supply. The anode is usually the metal to be plated (assuming that the metal will corrode in the electrolyte). For ease of operation, the metal is in the form of nuggets and placed in an inert metal basket made out non-corroding metal (such as titanium or stainless steel).
The cathode is the workpiece, the substrate to be plated. This is connected to the negative terminal of the power supply. The power supply is well regulated to minimize ripples as well to deliver a steady predictable current, under varying loads such as those found in plating tanks.
As the current is applied, positive metal ions from the solution are attracted to the negatively charged cathode and deposit on the cathode. As a replenishment for these deposited ions, the metal from the anode is dissolved and goes into the solution and balances the ionic potential.
In the case of materials such as gold, the anode is not sacrificial (gold does not dissolve easily !), but it is made out of material that does not dissolve in the electrolyte, such as titanium. The deposited gold comes out of the solution. Plating is an oxidation-reduction reaction, where one material gives up electrons (gets oxidized) and the other material gains electrons (gets reduced). The anode is the electrode at which oxidation occurs, and the cathode is the electrode at which reduction occurs.
Theory of Electroplating
Plating is governed by Faraday's Laws that state:
The weight of a substance formed at an electrode is proportional to the amount of current passed through the cell.
The weights of different substances produced at an electrode by the same amount of current are proportional to their equivalent weights.
Equivalent weight in an oxidation-reduction reaction is = molar weight of the compound / algebraic change in oxidation number of the atom that is oxidized or reduced.
e.g. In the reaction
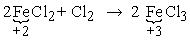
Fe valency (oxidation number) goes from +2 to +3, a change of +1. Therefore and equivalent of FeCl2 as a reducing agent is equal to 1 mole or 126.75 g
However in the reaction
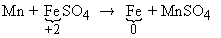
Here Fe (+2) is reduced to Fe (0).
The equivalent weight of is 75.96 g which is
= molar weight/change in valency.
Some background:

Thus 1 Faraday of charge is equivalent to one mole of electrons being liberated at the anode. (Do not confuse this with Farad, a unit of electrical capacitance).
In the case of AgNO3, 1 mole of Ag or 107.870 g of Ag is deposited when 1 Faraday of charge passes from anode to cathode.
In the case of CuSO4, 1/2 mole of Cu or 31.77 g of Cu is deposited when 1 Faraday of charge goes through the system.
In the case of AgCl3, 1/3 mole of Au or 65.656 g of Au is deposited when 1 Faraday of charge goes through the system.
Note that in each of the above cases the moles are different depending on the valencies and the actual reaction taking place. The atomic weight of each element can be obtained from the periodic table.
Thus 100 Ampere-hours in a CuSO4 solution will plate according to the following formula:
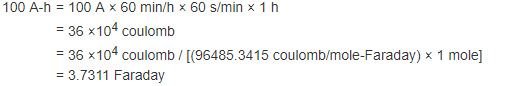
Since 1 Faraday will deposit 31.77g of Copper, 3.7311 Faraday will deposit 118.54 g of Copper.
Based on the mass of the metal deposited, the thickness can be calculated for objects of known surface area, because
Mass deposited = Density of the metal × Surface area × thickness.

