Common Conversion Coatings
Common conversion coatings processes are briefly discussed in this section, including oxide coatings, phosphate coatings, and chromate coatings.
Oxide Coatings: The oxide coatings are in fact corrosion products which is a thin, usually less than 2.5 µm (.00001 in) oxide with good adhesion. The oxide treatments are done by heat, chemicals, or electrochemical reactions.
Gun-bluing-type oxidations are done by heating the metals, generally steel, at 370ºC (700°F) in a steam atmosphere. An oiled gun bluing provides some atmospheric corrosion resistance, but little protection on wear and other corrosion.
Chemical baths produce coatings similar to a gun bluing coating by immersion techniques.
Black oxide treatments are done by proprietary chemicals. Some pastes can be rubbed on surfaces to produce similar results. Black oxide can be applied on steel, copper, and most stainless steel.
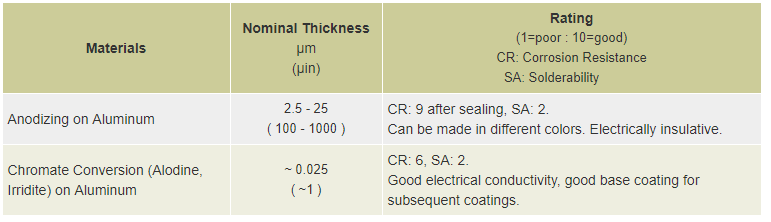
Anodizing is produced by electrochemical conversion. The anodizing process, usually performed on aluminum for protection and cosmetic purposes, builds up both on the surface as well as into the metal. Thin coatings, 2 µm to 25 µm (100 µin to 1000 µin) can be coated on most aluminums. Thick coatings from 25 to 75 µm (1000 to 3000 µin) are more durable and abrasion resistant than above chemical conversion oxide coatings. This oxide layer can be made in different colors depending on the post chemistries that are employed. The anodized parts are quite durable and do not tarnish and maintain their cosmetic appearance for a long period of time. Anodized coatings are usually dielectric in nature.
Phosphate Coatings: Phosphate coatings are processes of chemical conversion on a metal surface to produce thin adherent phosphate compound coatings. The phosphate crystals formed on the surfaces of materials can be iron, zinc, or manganese phosphates. Among these phosphates, manganese phosphate is more suitable for wear applications. Phosphate coatings are usually applied to carbon steel, low-alloy steel, and cast iron. They can also be applied to zinc, cadmium, aluminum, and tin. Phosphate processes are hard to apply on high alloys for these alloys are likely immune to the phosphoric acid. In short, phosphating is one of the most useful non-metallic coatings.
Chromate Coatings: Chromate coatings, similar to phosphate coatings, are processes of chemical conversion. But the chromate coatings are formed by the reaction of water solutions of chromic acid or chromium salts. The coatings can be applied to aluminum, zinc, cadmium, and magnesium. The coatings usually have good atmospheric corrosion resistance. Chromate coatings are widely used in protecting common household products, such as screws, hinges, and many hardware items with the yellow-brown appearance.
Coating Considerations